As spring firmly establishes itself across the U.S. with warmer weather and increased daylight, healthcare workers have finally seen the number of seasonal influenza cases subside. This winter’s 2017-2018 flu season was one of the worst in recent memory, with the Center for Disease Control (CDC) reporting over 30,000 confirmed cases of the flu resulting in hospitalization this year. Ironically, this year marked the 100th anniversary of the Spanish Flu Pandemic of 1918, which infected an estimated 500 million people worldwide and killed anywhere from 20 to 50 million people, nearly a third of the world’s population at the time. Each year sees a new flu vaccine targeted at the viral strains predicted to be most prevalent in that season, but because the disease is a virus, it can undergo rapid mutations and render vaccines ineffective against many of the circulating strains. The U.S. Agency for International Development, along with the CDC, closely monitors the flu, along with a number of other viruses that have been identified as possible pandemic threats. With the knowledge that an influenza pandemic could be right around the corner at any time, pharmaceutical companies, research institutions, and government health entities are aiming to develop a universally-effective flu vaccine that will protect against existing strains of the influenza virus as well as any new strains that could develop.
Flu Vaccine Variants
Flu vaccines in the United States come in three variants. The egg-based vaccine has been around the longest, going back over 70 years. This technology involves injecting a live flu virus strain into a chicken’s egg and allowing the virus to replicate for a number of days, after which time the virus-containing fluid is extracted from the egg. The virus can either be killed for injectable flu shots or weakened and taken as a nasal spray. Cell-based flu vaccines, a second variety, use mammalian cells instead of chicken eggs to replicate the virus and extract and purify the viral antigen. Finally, the newest technology approved by the FDA in 2013 is the recombinant flu vaccine. Viral protein is isolated from a naturally-occurring flu virus and then combined with portions of another virus grown in insect cells. The recombinant virus is replicated in insect cells, then the protein is harvested and purified. The recombinant method takes the shortest amount of time to produce a flu vaccine and is the technology upon which a number of universal flu vaccine patents are based. At present, 69 patents for a universal flu vaccine exist worldwide, a recent example of which being US9688965B2, entitled “Recombinant neuraminidase and uses thereof”.
Source: ktMINE Patents App contains over 102 million patents & assignments.
Top Flu Vaccine Patent Owners
In the United States, commercially-available flu vaccines are produced by pharmaceutical companies in the private sector, often receiving sponsorship and/or funding from governmental organizations such as the National Institutes of Health (NIH). On May 8th of this year, a division of the NIH, the National Institute of Allergy and Infectious Diseases, announced its sponsorship of a global flu vaccine currently being produced by the Israeli-based company BiondVax Pharmaceuticals. This is not the first universal flu vaccine to be developed, however. Ten years ago, VGX International Inc. entered into a collaborative license agreement with Inovio Biomedical Corporation for the joint development of a universal flu vaccine to be available throughout Asia.
VGX International Inc. Collaborative License Agreement
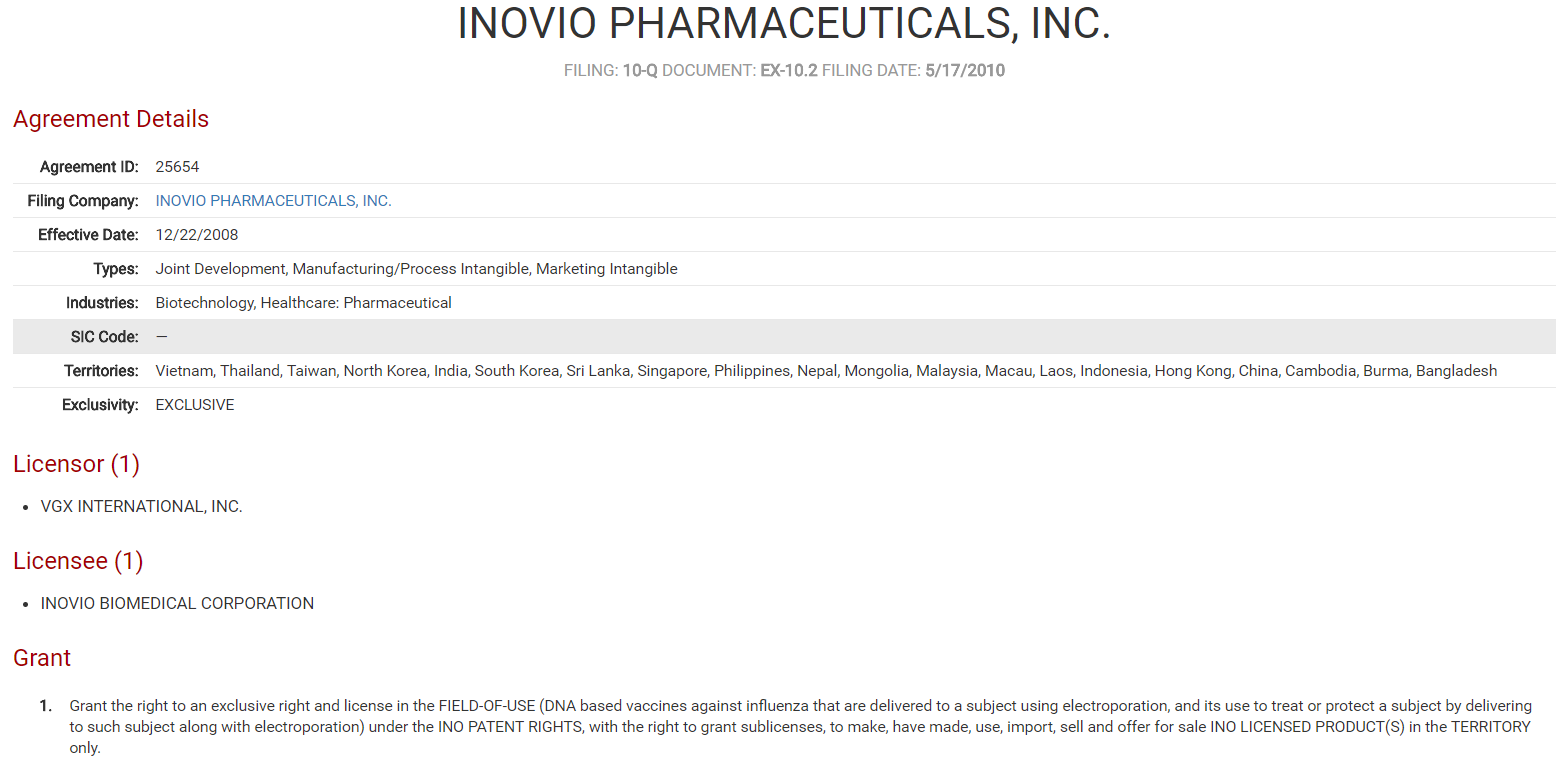
Source: ktMINE Agreements App contains over 120,000 full-text license agreements.
Patent applications in the field began in 2007, with the first grant coming in 2011. Currently, the five top owners for patents in the field include:
- Panacea Biotech Ltd (14)
- The Secretary of State for Health (9)
- The Wistor Institute for Anatomy and Biology (8)
- Georgia State University Research Foundation Inc. (4)
- Mogam Biotechnology Research Institute (4)
Universal Vaccine: Closer Than You Think
While the CDC recommends everyone over 6 months of age receive an annual flu vaccine, only about 1/3rd of Americans received it this year. Worldwide, a general apathy exists towards consistently getting vaccinated against the flu. A survey in Australia conducted this year found that about 50% of Australians did not plan on getting vaccinated this year, the most common reasons being disbelief in its effectiveness and “not getting around to it.” Regardless of these statistics, pharmaceutical companies are still investing heavily in the development of a universal flu vaccine that will provide protection against existing and future strains. If this vaccine has a higher efficacy rate (on average, seasonal flu vaccines are anywhere from 10-60% effective against contracting the illness), consumers may be more motivated to get one yearly. With governmental organizations and private-sector pharmaceutical companies working together, a universal flu vaccine is poised to come to market sooner rather than later.